Categories
Clear Filters
Envetec
Biohazardous Waste
Carbon Reduction
Recycling
GENERATIONS Operation & Requirements
Efficacy
Regulation
Chemical
Water
What is Envetec?
- Envetec is a wholly Irish-owned company with a global vision to create clean change. Envetec’s mission is to revolutionise how laboratories across the life science sector treat biohazardous waste, particularly recyclable single-use plastics, transforming how lab environments approach sustainability.
What Industries do you serve?
- GENERATIONS was designed to be suitable within all life science operations. Our current operations centre around five main industries.
What is biohazardous waste?
- Also called infectious waste or regulated medical waste (such as blood, body fluids, and human cell lines), biohazardous waste is waste contaminated with potentially infectious agents or other materials that are deemed a threat to public health or the environment.
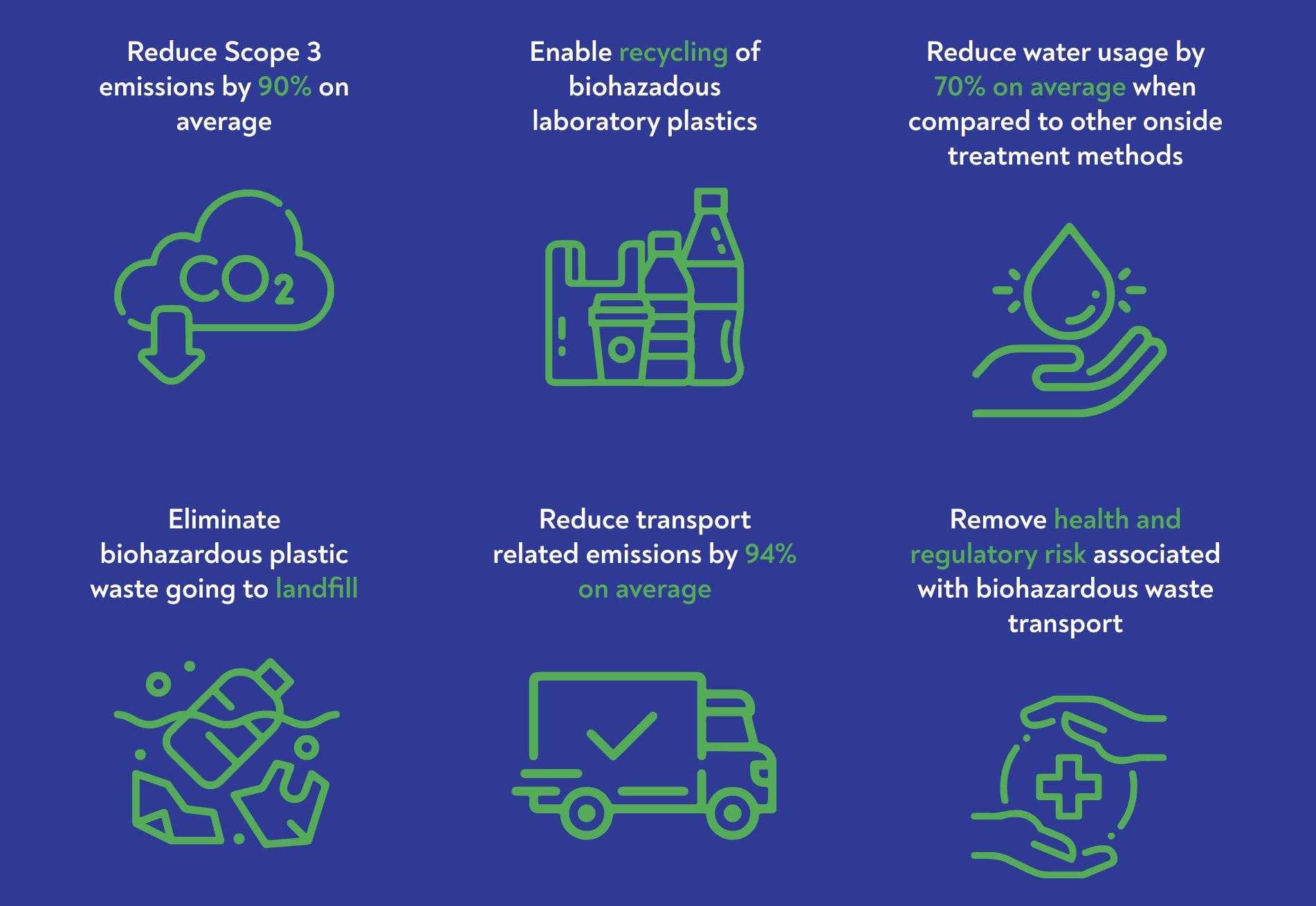
Has a carbon life cycle analysis (LCA) of the GENERATIONS technology been performed to compare the GENERATIONS process with traditional biohazardous waste treatment methods?
- To independently validate our technology, we requested Carbon Action, a London based independent third-party assessor of sustainability, to perform eight separate evaluations of the impact of the GENERATIONS technology on lowering carbon emissions across sites in the U.S., U.K. and Ireland.
- The study benchmarked the life cycle emissions of processing a fixed amount of biohazardous waste against different waste treatment methodologies including incineration, landfill and autoclaving.
- The independent data shows GENERATIONS’ ability to reduce Green House Gas emissions by 90% on average, compared to commonly used treatment approaches.
- The GHG comparison assessment report also highlighted additional benefits that extend beyond emissions while impacting the treatment processing chain. These benefits include the elimination of road congestion and the avoidance of transporting biohazardous waste; enhanced circularity with an easily recycled end product; and reduction in energy usage by displacing power for steam and electricity.
Traditionally biohazardous plastics cannot be recycled, how is the output of the GENERATIONS process different?
- Historically, the only reason that biohazardous material cannot be recycled is because it is biohazardous and treatment methods such as large-scale autoclaving and incineration do not produce recyclable end product.
- The globally accepted efficacy protocol for the treatment of biohazardous waste is the STAATT III (4log10 reduction of pathogens). GENERATIONS achieves the superior STAATT IV (6log10 reduction of pathogens) by combining our unique shredding technology and our proprietary, organic, biodegradable disinfectant solution.
- As our process is non-thermal the treated plastic are not affected by thermal degradation seen in autoclaves etc. meaning it is more desirable for recyclers.
Does the GENERATIONS System affect the properties of polymers and/or the ability to be reprocessed?
- In repeated analysis, material treated with Envetec’s GENERATIONS technology showed no discernible difference of modulus (rigidity) to standard plastic material that is utilised in recycling processes.
How can GENERATIONS contribute to a circular economy?
- GENERATIONS is an essential link in enabling the recycling of biohazardous waste including polymers and metals. Polymers treated by GENERATIONS are suitable to be recycled for reuse.
How does the system work?
- Envetec GENERATIONS has a robust, non-thermal shredding process which macerates waste products into confetti-like flake. Waste is treated with an organic, biodegrable chemical compound which is commonly used as a disinfectant in healthcare, food and water treatment sectors. The shredding process ensures that all parts of the waste are subjected to the chemical treatment process.
What can GENERATIONS treat?
- GENERATIONS is designed and validated to treat a wide range of biohazardous waste such as agar plates, IV tubing, sharps, blood tubes, bioreactors etc.
- We perform preliminary testing on potential clients’ material to ensure that their waste loads are suitable for treatment via GENERATIONS.
Are there any materials that should not be processed in a GENERATIONS?
- GENERATIONS is not intended to process the following:
- Pharmaceutical waste
- Anatomical waste (human and animal)
- Large metallic waste (Prosthetics etc.)
- Flammable or explosive substances
- Radioactive material
What are the installation requirements of GENERATIONS?
- As GENERATIONS is a non-thermal technology the installation requirements are similar to those of most lab-based technologies. The initial requirements for GENERATIONS include:
- 3-phase power supply
- A water supply line
- Adequate standard drainage
- An ethernet connection
- Dimensions
- Height 1.91 metres
- Length 3.57 metres
- Depth 1.65 metres
- Weight 2265.5 kgs
For a full list of installation requirements reach out to our team
How easy it is to use?
GENERATIONS is designed with ease of operation in mind to ensure efficiency. The user-friendly interface allows a treatment to be selected in 3 simple clicks
-
- Step 1: Biohazardous waste in bags or rigid containers is loaded into the GENERATIONS treatment chamber.
- Step 2: The user initiates the validated treatment process by pressing the “run” button.
- Step 3: As the treatment process begins, GENERATIONS automatically adds the organic, biodegradable chemical and water to the treatment chamber as shredding commences.
- Step 4: Upon completion, the treatment chamber rotates, transferring the processed material to a dewatering auger, which separates solid and liquid material. The flake is now ready for recycling and the safe, treated effluent is sent into the facility’s water management system.
- Check out this short video showing how easy it is to begin a treatment cycle with GENERATIONS
How do you prove the output is noninfectious?
- GENERATIONS disinfection process is validated and surpasses STAATT IV testing requirements which confirms a consistent inactivation of 6log10, 100x more efficient than the recommended global standard for autoclave and rotoclave technology.
- Envetec’s GENERATIONS treatment process has been efficacy tested and received approval form U.S. EPA, New York State Department of Health and satisfies all EA requirements.
- For regions in which regular validation is required, GENERATIONS hosts a patented, in-built validation chamber that allows for validation to be carried out during live waste cycles.
How is validation carried out on GENERATIONS?
- Envetec has designed a unique validation process that verifies efficacy of treatment during live processing of waste.
- The novel system reduces the need for validation related down time, reduces operator contact with potentially hazardous agents and increases the accuracy of process validation.
How often is validation required?
- Validation depends on the territories that you operate in. Contact our team using the form provided to discuss the requirements in your region.
In the U.S. market, are any additional permits required to treat my company’s biohazardous waste with GENERATIONS?
- The U.S. EPA is the competent authority with regard to the approval for use of the Envetec’s organic, biodegradable chemical, Vigor-Ox. It has granted approval for its use conditional on its use within Envetec GENERATIONS.
- The use of Envetec GENERATIONS in the treatment of regulated medical waste is subject to state provisions which can differ in their oversight of facilities, but approval will refer to the efficacy studies as submitted to US EPA.
- In the U.S. market, Envetec GENERATIONS is considered an “Alternative Technology” in the treatment of Regulated Medical Waste and accordingly as an on-site treatment technology, requires the approval of the relevant authorities within whose jurisdiction the facility operates. Our experience to date has been that approval for use of the alternative technology, draws heavily on the efficacy studies submitted to the US Environmental Protection Agency.
What chemical does GENERATIONS use for treatment?
- The chemical composition used in GENERATIONS to treat the biohazardous waste, was approved in February 2002 by the EPA in the US, and is an organic, biodegradable antimicrobial agent.
- US EPA approval was based on extensive review of treatment efficacy of the organic, biodegradable chemical when used in GENERATIONS for the treatment of Regulated Medical Waste.
- The treatment chemical utilises peracetic acid which is one of the most common chemicals used in water treatment and the food industry in the world including the EU.
- Safety Data Sheets outlining the chemical composition of products used are made available to all clients.
What concentration of chemical is required for the GENERATIONS treatment process?
- The concentration of chemical used depends on the material being treated. Waste loads such as primary blood tubes or blood bags that have a high biological load and require a higher concentration of our organic, biodegradable chemical.
- As part of our R&D and engineering trials Envetec has validated different waste streams with concentrations of chemical ranging from 1% to 3%. On receiving the list of materials that a laboratory wishes to treat, Envetec will advise on the concentration of chemical to be used. The system is capable of having multiple programs for different waste streams.
- The objective of using different concentrations of chemical is to guarantee the efficacy of the system in terms of biological kill of different waste loads.
What is the environmental footprint of Envetec’s proprietary chemical?
- The carbon footprint of the organic, biodegradable chemical used in the GENERATIONS process are accounted for in the calculation for GENERATIONS Emissions (including embedded emissions) and considered as scope 3 emissions. The approach adopted in this accounting is as set out in ISO 14064-1 “Specification with guidance at the organizational level for quantification and reporting of greenhouse gas emissions and removals.”
How is effluent from the GENERATIONS process handled?
- The treatment cycle involves the addition of 20 liters of water plus 200 – 600 milliliters of Envetec’s organic, biodegradable chemical, followed by a further addition of 20 liters of water as a standard rinse cycle. The chemical is comprised of Peracetic Acid and Hydrogen Peroxide which rapidly degrades in the environment and does not bioaccumulate.
- The GENERATIONS process leaves wastewater at a pH between 6.5 – 7.5, this can include treatment via a neutralizing step if required.
- Post-treatment, the effluent is filtered through a filtration membrane to remove smaller particles and other semi-aqueous substances that may be in suspension in the effluent stream, before being released into the facility’s water management system.